Application of Electronic Effect
Application of Electronic Effect: Overview
This Topic covers sub-topics such as Bond Order, Bond Length, Bond Parameters, Acidic Strength of Organic Compounds, Basic Strength of Organic Compounds, Basic Compounds and, Effect of Electronegativity on Basic Strength of Compounds
Important Questions on Application of Electronic Effect
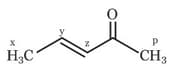
The abstraction of proton will be fastest from which carbon in the following compound?
Which of the following has longest C-O bond:
Rank the following compounds in order of decreasing acidity of the indicated hydrogen :
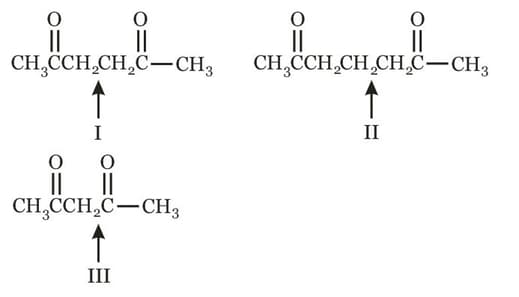
The increasing order of basic strengths in their aqueous solutions is:
Which of the following compound do not give the protonation reaction readily?
Arrange in order of increasing acidic strength

Amongst the following, the most basic compound is
Identify the organic bases from the given options.
Which one of the following is not an organic base?
Describe the basic character of alcohols and amines.
Give some examples of organic bases.
Both amines and alcohols are Lewis bases.
The bond order of individual carbon-carbon bonds in benzene is:
Among the following oxoacids, the correct decreasing order of acid strength is :
The correct order of increasing acid strength of the compounds
(a)
(b)
(c)
(d) 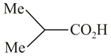 is?
is?
Among the following acids which has the lowest value?
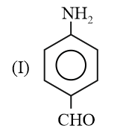
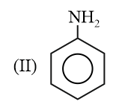
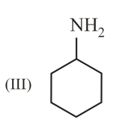
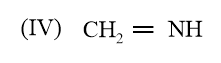
The correct order of bond length in the above compound is:
The correct order of bond length in the following compound is:
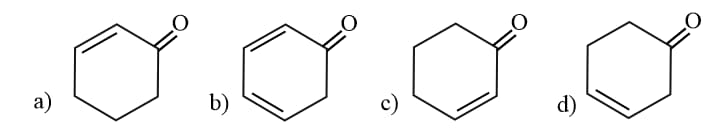
The acidity of
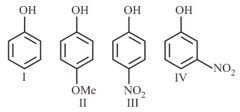
Follows the order
The order of strengths of the following carboxylic acids is
a)
b)
c)
d)
